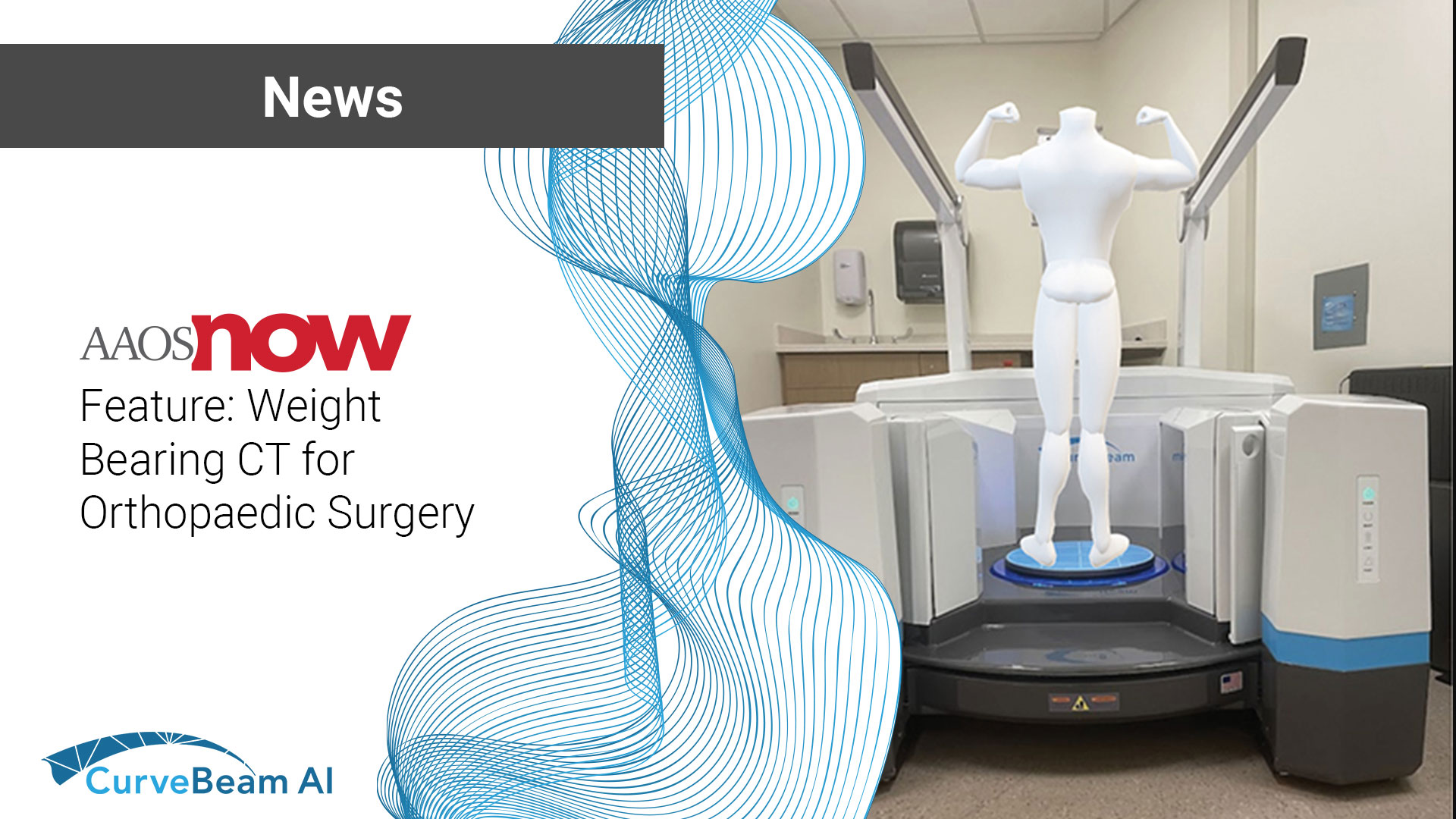
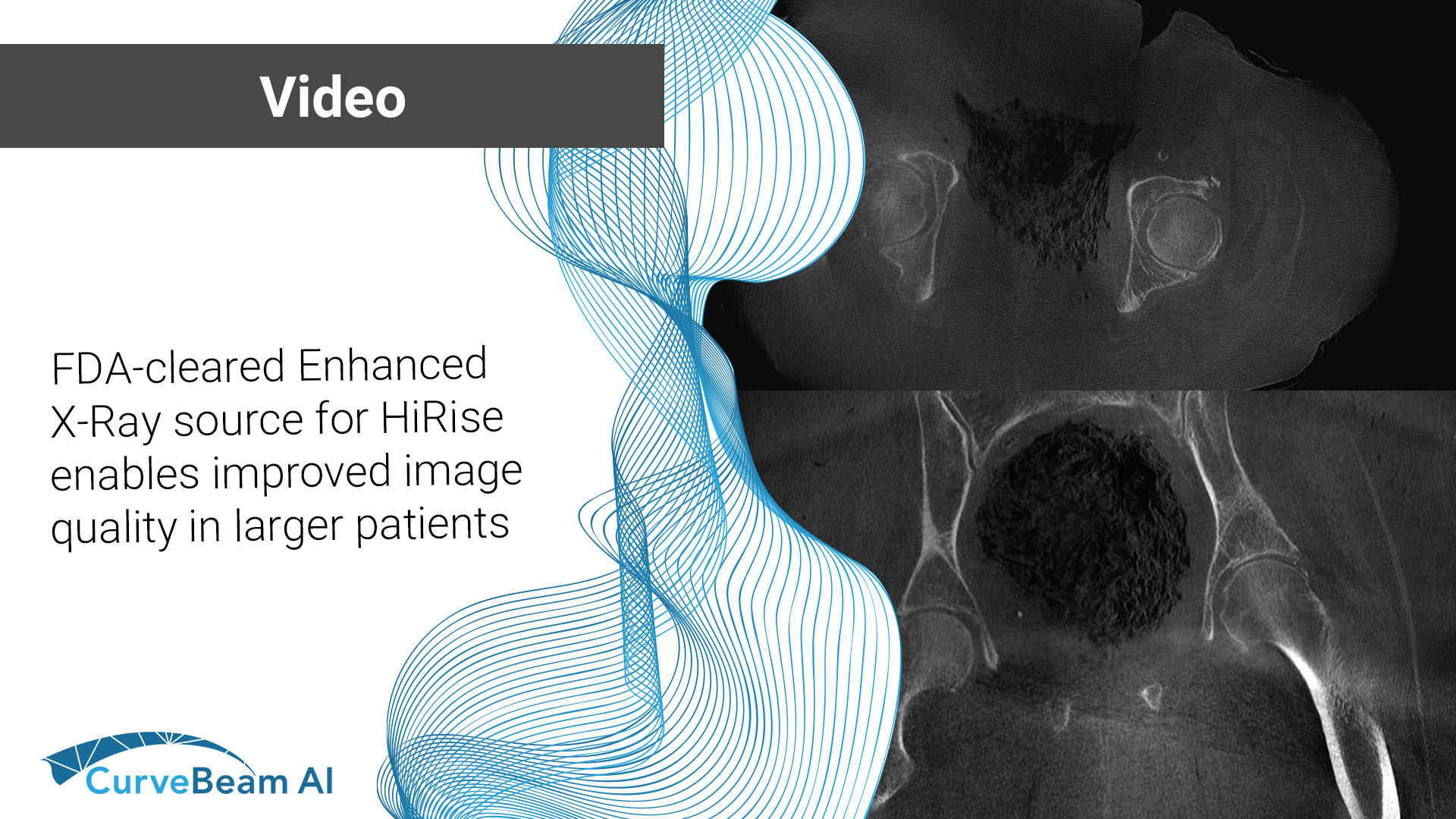
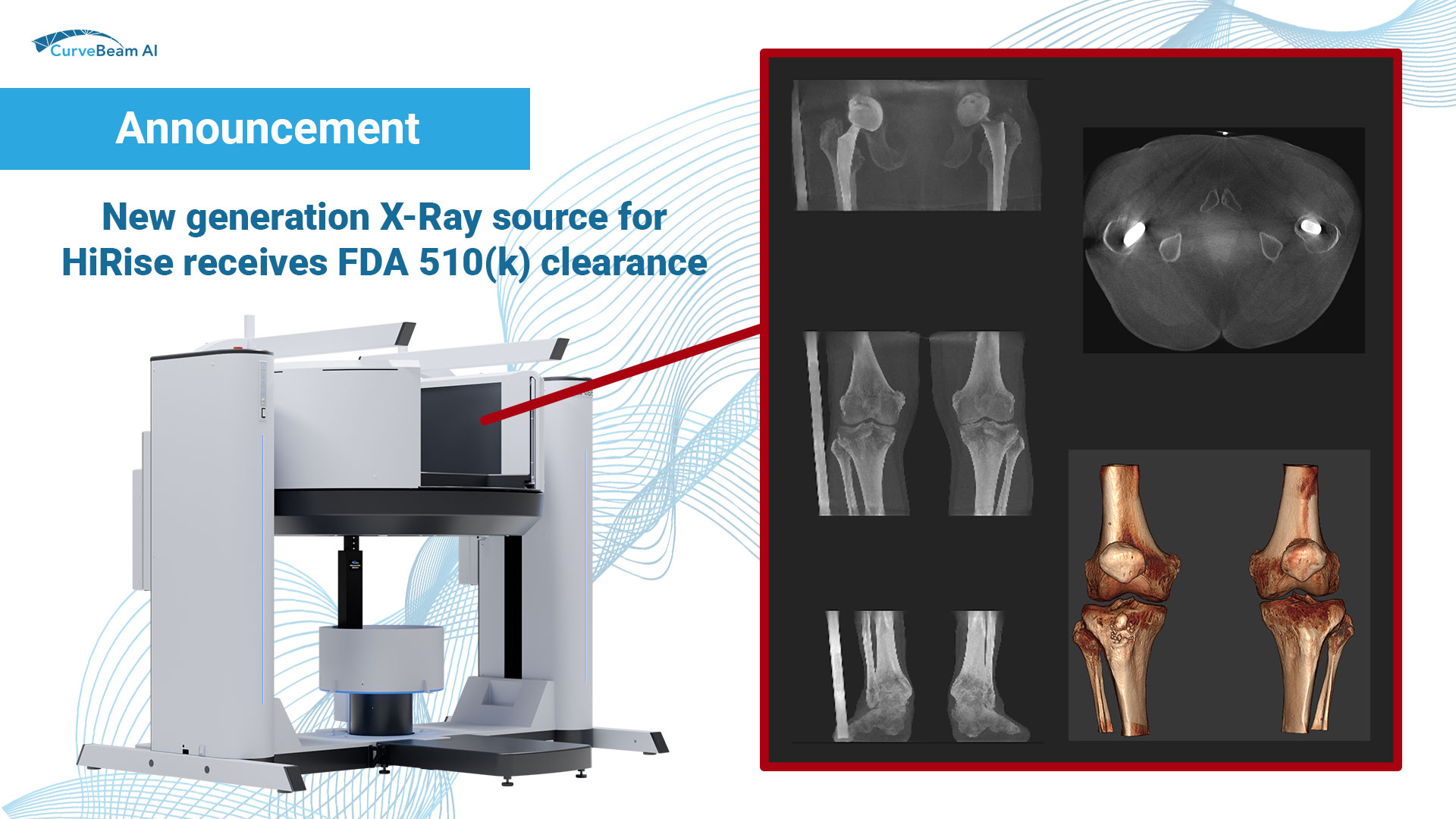
The August/September 2024 issue of AAOS Now includes the first of of a two-part series…

MedShape And CurveBeam Announce Initiation of Joint Prospective Clinical Study
Research will evaluate the efficacy of a sustained dynamic compression device using longitudinal weight-bearing CT
ATLANTA (May 10, 2021) – MedShape, Inc., the orthopedic industry leader in promoting active, adaptive healing technologies, and CurveBeam, the leader in weight-bearing CT (WBCT) imaging, jointly announced today the initiation of a new prospective clinical study with the Orthopedic Foot & Ankle Center (Worthington, OH). The study, titled “Tibiotalocalcaneal Arthrodesis Utilizing a Sustained Dynamic Compression Intramedullary Nail with Longitudinal Weight-Bearing CT Analysis” (NCT04831645) will evaluate the efficacy of MedShape’s DynaNail® TTC Fusion System using CurveBeam’s LineUPTM WBCT system. Both MedShape (Booth #1115) and CurveBeam (Booth #414) will be exhibiting at the American College of Foot & Ankle Surgeons (ACFAS) Annual Scientific Conference next week in Las Vegas.
Since its launch in 2013, the DynaNail has emerged as a market leader in internal fusion devices as the only intramedullary nail that provides sustained dynamic compression. Its patented internal nickel titanium (NiTiNOL) Compressive Element actively adapts to bone resorption and other changes during bone healing, a feature that can be tracked post-operatively on radiographs. While MedShape has several ongoing clinical trials evaluating the efficacy of a sustained dynamic compression nail,1 this 45-patient study marks the first aimed at correlating the NiTiNOL Element recovery and three-dimensional fusion assessment with a patient’s functional improvement.
“There is a gap in the clinical literature around the spatiotemporal development of the fusion mass and the factors that affect this process,” said Ken Dupont, PhD and MedShape Director of Clinical Research. “With this study, we will be able to assess when and where specifically bone growth occurs and compare this growth pattern amongst different patient groups with various challenging co-morbidities. We already know that sustained dynamic compression can lead to improved fusion results2, but this new data could help optimize the compression performance in future devices.”
By using CurveBeam’s low dose LineUP WBCT System, CT scans will be obtained at 5 post-operative intervals (up to 12 months), allowing for more longitudinal temporal tracking of the fusion process than would be permitted with traditional CT systems. The weight-bearing CT protocol also allows for a more accurate three-dimensional reconstruction of the fusion site that can then be compared with two-dimensional radiographs.
The surgeons at the Orthopaedic Foot & Ankle Center represent the prime clinical investigators to participate in the study, with a proven track record in producing high-quality research aimed at improving patient care as well as extensive experience with both the DynaNail and LineUP systems. Mark Prissel, DPM, one of the clinical investigators, will be sharing more details around the study design and objectives in a presentation at the CurveBeam ACFAS booth on May 20th at 9:40am.
“Achieving successful outcomes in patients who undergo TTC arthrodesis has proven challenging to date,” stated Dr. Prissel. “We hope the results of this study will not only demonstrate the clinical advantages of dynamic compression fixation, but also provide further fundamental knowledge with serial WBCT imaging that will allow surgeons to tailor their post-operative protocols to better treat and address individual patient needs.”
About MedShape Inc:
MedShape Inc. is a privately held medical device company working to develop and commercialize a portfolio of surgical solutions that use its patented active, adaptive healing technologies to address the increasing demand for improved joint fusion, sports medicine, and musculoskeletal trauma products. For more information, visit: http://www.medshape.com.
About CurveBeam Inc:
CurveBeam researches, designs and manufactures cone beam CT imaging systems for the orthopedic specialties. CurveBeam’s corporate headquarters is located in Hatfield, Pennsylvania. The company was founded in 2009 and is privately owned and operated. For more information, visit: https://www.curvebeamai.com.
1https://clinicaltrials.gov/, Keyword: MedShape; Identifiers: NCT02324907, NCT03686241, NCT04784273, NCT03747952, NCT04338607, NCT04784156.
2Steele JR, Easley ME, Nunley JA, Adams SB, et al. Comparison of Tibiotalocalcaneal Arthrodeses Using a Sustained Dynamic Compression Nail Versus Nondynamized Nails. Foot & Ankle Spec, 2020; 13(3): 193-200.
DynaNail is a registered trademark of MedShape, Inc. LineUP is a trademark of CurveBeam.