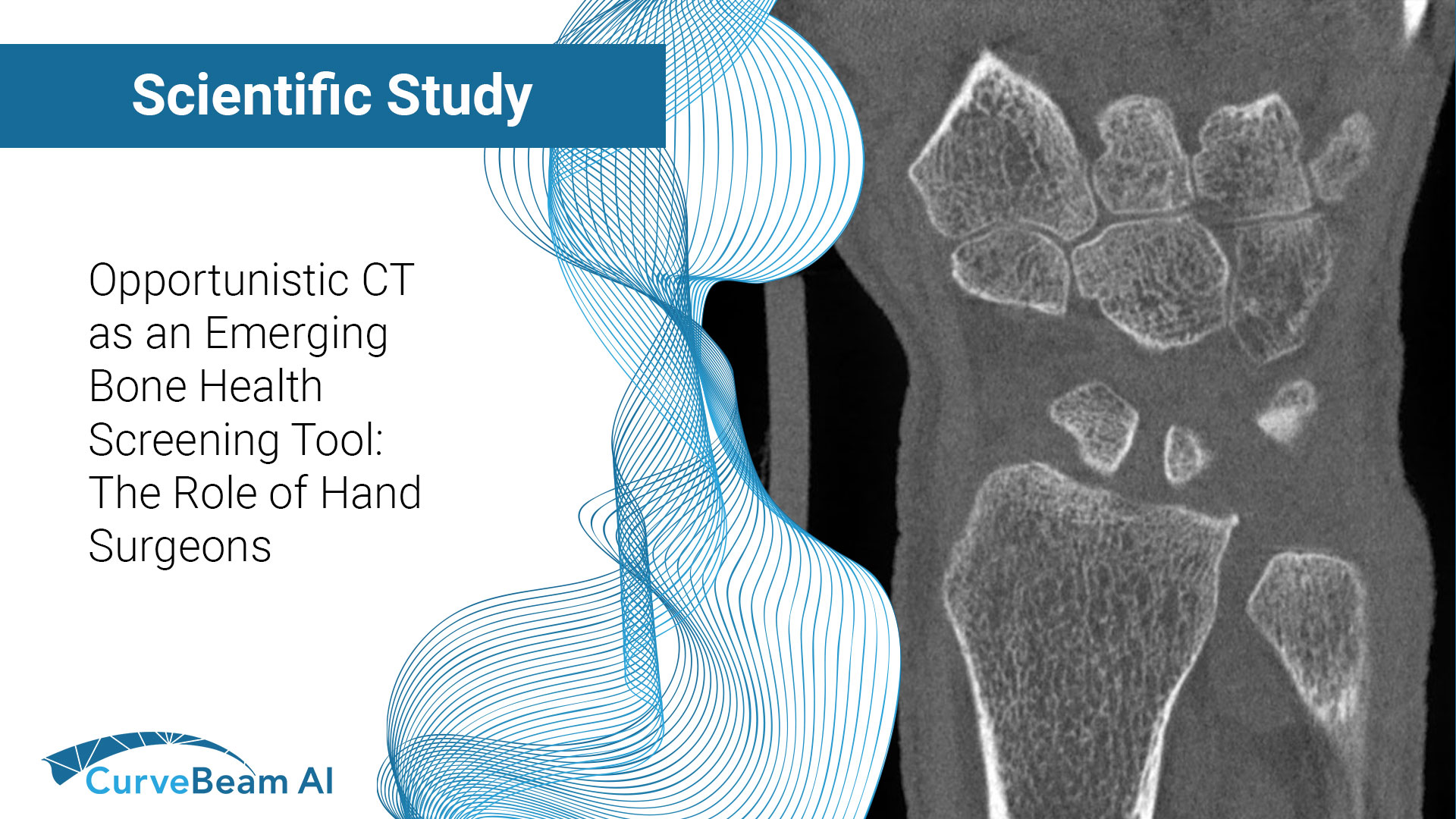
Fragility fractures are often the first visible sign of underlying osteoporosis but too often, they…

CurveBeam Announces FDA 510(k) Clearance for InReach Cone Beam CT Imaging System for the Upper Extremities
 May 8, 2017 – Warrington, Penn. – CurveBeam announced it has received FDA 510(k) clearance for the InReach, a Cone Beam CT imaging system primarily designed for the hand, wrist &elbow; & lower extremities in non-weight bearing position.
May 8, 2017 – Warrington, Penn. – CurveBeam announced it has received FDA 510(k) clearance for the InReach, a Cone Beam CT imaging system primarily designed for the hand, wrist &elbow; & lower extremities in non-weight bearing position.
The InReach is an ultra-compact CT scanner that provides high-contrast 3D datasets of bony anatomy, which could potentially replace radiographs as a first line of diagnosis.
The InReach is ideal for the point-of-care because of its small footprint, its self-shielded design, and standard power requirements. Point-of-care 3D imaging allows for faster diagnosis and more accurate treatment plans.
“The InReach will revolutionize the speed and accuracy of assessment of upper extremity conditions that specialists have traditionally found challenging to diagnose with plain X-Ray, such as scaphoid fractures,” said CurveBeam President & CEO, Arun Singh. “The InReach continues the company’s mission to elevate advanced diagnostic imaging capabilities to enhance orthopedic care.”
The InReach is designed with patient comfort in mind. Patients’ hand, wrist or elbow is positioned in a height-adjustable bore while in standing or sitting position. The unit can also accommodate non-weight bearing, lower limb imaging. Scan times are less than 30 seconds.
The InReach device is supplemented by CubeVue, CurveBeam’s custom visualization software. CubeVue gives orthopedic specialists unprecedented access to multi-planar slices and vivid 3D renderings of the anatomy previously not easily accessible to specialists. CubeVue’s Insta-X feature provides Digitally Reconstructed Radiographs, potentially eliminating the need for radiographic exams altogether.
The InReach is the second extremity CT imaging system CurveBeam has introduced to the market.
CurveBeam is the leader in Weight-Bearing extremity CT imaging, starting with the introduction of its pioneer product, the pedCAT, in 2012. The pedCAT is the only CT system that allows for bilateral, true weight bearing imaging of the lower extremities. Since 2012, the pedCAT has been integrated into leading foot & ankle orthopedic and podiatric practices around the world.
CurveBeam is currently developing its next generation multi-extremity device, the LineUP, which will provide bilateral Weight-Bearing images of the knees in addition to feet, as well as hand, wrist & elbow. CurveBeam anticipates the LineUP will be submitted for FDA review by July, 2017.