Hatfield, PA — July 1, 2025 — CurveBeam AI, a global leader in weight bearing…
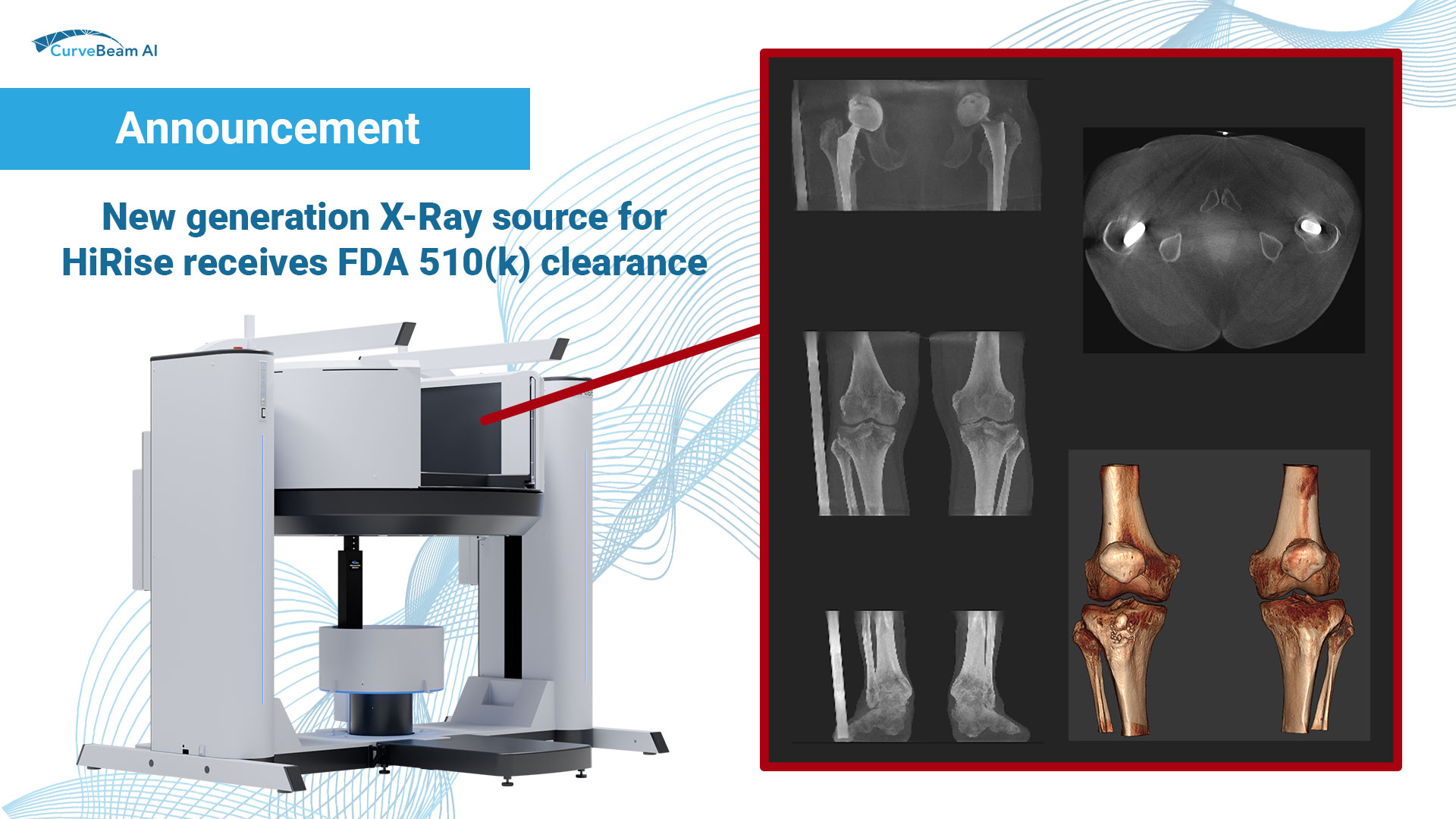
New generation X-Ray source for HiRise receives FDA 510(k) clearance
CBAI is proud to announce it has received FDA 510(k) clearance for enhancements to the HiRise system, including a new generation X-Ray source that is capable of delivering more power than the previous model. This new generation X-Ray source enables improved image quality in larger patients, a greater range of energy settings, and a broader selection of vendor-specific protocols.